

hLL-37 17–29 is also the region within hLL-37 showing the highest sequence similarity to PSMα3 (Supplementary Fig. Although not directly detected in vivo, hLL-37 17–29 can be cleaved from hCAP-18 or LL-37 by either proteinase K or staphylococcal peptidase I on its N-terminal side, and by trypsin on its C-terminal side. The hLL-37 17–29 fragment was suggested to serve as the active core of the AMP, showing a different spectrum of antibacterial activity as compared to the full-length protein and other fragments, including being the shortest LL-37 fragment retaining anti-viral activity 36, 39, 40, 41. The antibacterial L元7 17–29 forms supra-helical fibrils Structure-guided mutagenesis supported a plausible active fibril arrangement. Here we demonstrate that the active core peptide of hLL-37, containing residues 17–19 (hLL-37 17–29), formed supra-helical highly stable fibrils that interact with bacterial cells. Bacterial proteases can too cleave LL-37, supposedly for degradation or to release virulence factors 36. Some hLL-37 fragments show a diverse array of selectivity against microbial strains, and additional functions within the immune system 34, 37, 38. Both PSMα3 and hLL-37 are cleaved in vivo into active truncations with diverse activities 32, 33, 34, 35, 36. Fibrillation of LL-37 was found critical for binding DNA and affecting receptors in the immune system 31. 1), a hCAP-18 protein cleavage product which plays an important role in the first line of defense against pathogens 28, which is also known to self-assemble 29, 30. Although originating from different organisms, PSMα3 display sequence similarity with human LL-37 (hLL-37) (Supplementary Fig. Moreover, this architecture offers a scaffold for the design of various supramolecular nanostructures 1, 8. Overall, PSMα3 provides a link between toxic activities against human and bacterial cells and unique helical amyloid fibrils 22, 23. PSMα3 is toxic to human cells, and some of its truncations and mutants show antibacterial activity 25, 26, 27. These fibrils are composed entirely of α-helices that stack perpendicular to the fibril axis into mated “sheets”, just as the β-strands assemble in amyloid cross-β fibrils 24. Our previous findings demonstrated cross-α amyloid fibrillation of the cytotoxic phenol-soluble modulin α3 (PSMα3) peptide secreted by the pathogenic bacterium Staphylococcus aureus 22, 23.
Correspondingly, recent evidence of antimicrobial properties among some human amyloids suggests a potential physiological role of proteins otherwise known as pathological 17, 18, 19, 20, 21. Certain AMPs assemble into well-ordered fibrils that resemble amyloids 11, 12, 13, 14, which are proteins known to form cross-β fibrils composed of tightly mated β-sheets, and have been associated with neurodegenerative and systemic diseases 15, 16. AMP self-assembly bears functional relevance and can enhance antimicrobial activity 10. Antimicrobial peptides (AMPs) are canonical components of the innate immune system of many organisms 9. One such application is addressing the urgent need to fight microbial aggressive, resistance, infections using materials which allow oral bioavailability, stability in harsh conditions, and long shelf-life. The assembly of basic biological molecules into filamentous structures provides ample opportunities to design bioinspired materials for medical and technological applications 1, 2, 3, 4, 5, 6, 7, 8. The findings expose a protein fibril which performs a biological activity, and offer a scaffold for functional and durable biomaterials for a wide range of medical and technological applications. This argues helical, self-assembling, basic building blocks across kingdoms of life and points to potential structural mimicry mechanisms. LL-37 17–29 resembles, in sequence and in the ability to form amphipathic helical fibrils, the bacterial cytotoxic PSMα3 peptide that assembles into cross-α amyloid fibrils. Structure-guided mutagenesis analyses supports the role of self-assembly in antibacterial activity. LL-37 17–29 correspondingly forms wide, ribbon-like, thermostable fibrils in solution, which co-localize with bacterial cells.
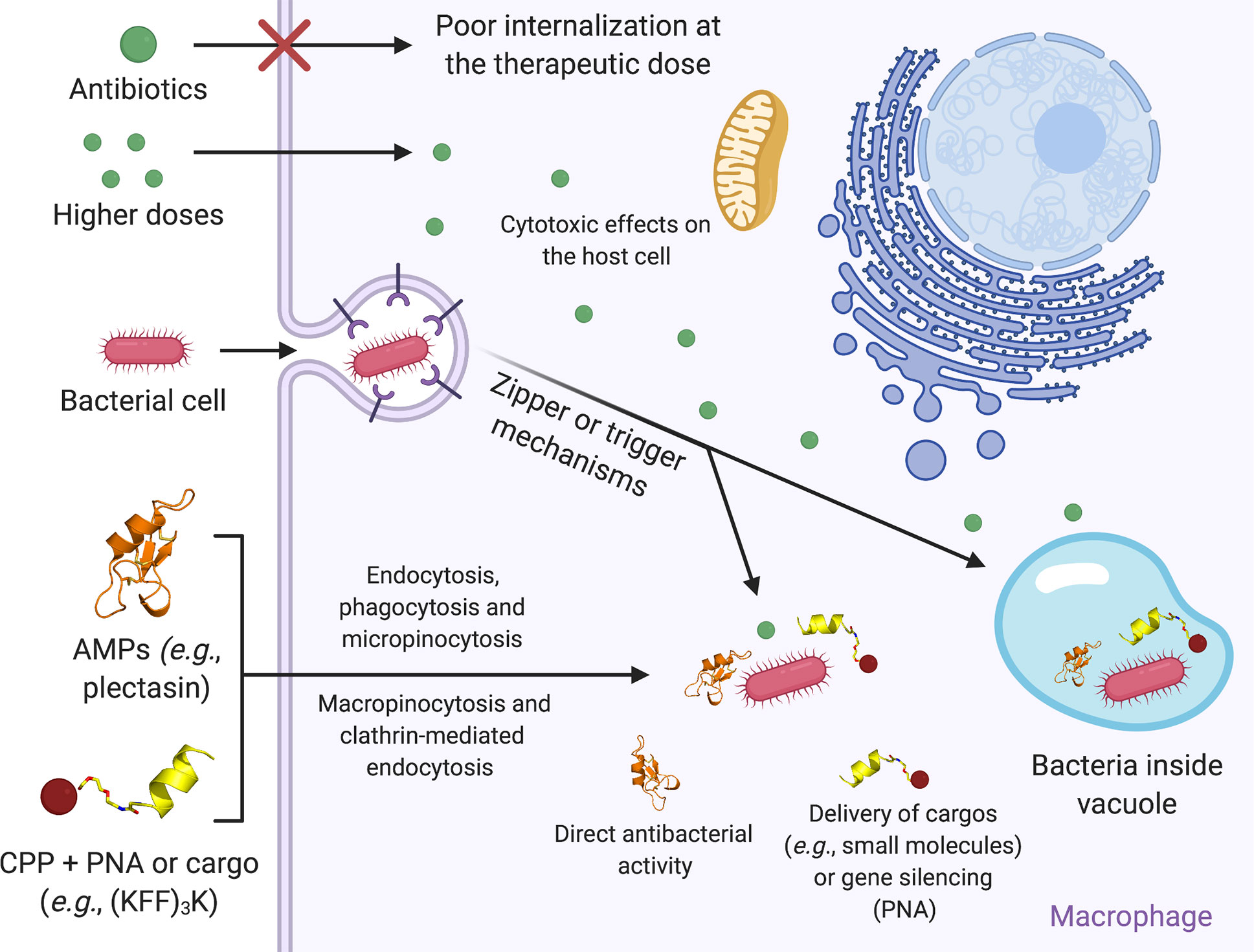
The surface of the fibril encompasses alternating hydrophobic and positively charged zigzagged belts, which likely underlie interactions with and subsequent disruption of negatively charged lipid bilayers, such as bacterial membranes. Here, we demonstrate the self-assembly of the antimicrobial human LL-37 active core (residues 17–29) into a protein fibril of densely packed helices.


 0 kommentar(er)
0 kommentar(er)
